Need information on DTI Cipro registration in South Africa? Focus on your company’s unique requirements. Begin by understanding the specific documentation needed for your business type; this varies significantly depending on whether you’re a sole proprietor, partnership, or company.
Directly contact the Department of Trade, Industry and Competition (the DTI) for the most up-to-date forms and instructions. Their website provides helpful guides, and their phone support is surprisingly responsive. Prioritize accurate completion of all forms; incomplete submissions cause delays.
Remember: The entire process involves multiple steps, including registration and potentially compliance certifications. Expect a timeframe of several weeks, though this can change depending on current workload and specific company circumstances. Proactive communication with the DTI throughout the process helps prevent problems and ensures timely completion.
Seek assistance from a registered business consultant if you encounter complexities or need help navigating the regulations. Many offer streamlined support and can significantly reduce your administrative burden. This investment saves time and ensures accuracy, ultimately benefiting your business’s success.
- Dti Cipro South Africa: A Comprehensive Guide
- Understanding CIPRO
- Key Steps for DTI Interaction
- Common Issues and Solutions
- Contacting CIPRO
- Understanding the DTI’s Role in Ciprofloxacin Regulation
- Registration Process
- Post-Market Surveillance
- Import and Export Control
- Key Regulatory Aspects
- Reporting Adverse Events
- Ciprofloxacin’s Availability and Accessibility in South Africa
- Finding Ciprofloxacin: Practical Advice
- Addressing Accessibility Gaps
- Pricing and Affordability of Ciprofloxacin in South African Pharmacies
- Factors Influencing Ciprofloxacin Prices
- Tips for Affordable Access
- Understanding Prescription Requirements
- Common Uses and Prescribing Practices of Ciprofloxacin in South Africa
- Respiratory Infections
- Other Applications and Considerations
- Prescribing Guidelines and Monitoring
- Safety Concerns and Side Effects Associated with Ciprofloxacin Use
- Reporting Adverse Events Related to Ciprofloxacin in South Africa
Dti Cipro South Africa: A Comprehensive Guide
Check the official DTI website for the most up-to-date information on CIPRO applications and requirements.
Understanding CIPRO
CIPRO, or the Companies and Intellectual Property Registration Office, handles company registrations and intellectual property rights in South Africa. The Department of Trade, Industry and Competition (dti) oversees CIPRO.
- Company Registration: CIPRO registers various business structures, including close corporations, private companies, and public companies. You’ll need specific documentation depending on the structure.
- Intellectual Property: This includes trademarks, patents, and designs. Registering your intellectual property protects your creations from infringement.
Key Steps for DTI Interaction
- Identify your needs: Determine whether you require company registration or intellectual property protection.
- Gather necessary documents: This varies greatly depending on the specific service needed. The CIPRO website provides detailed checklists.
- Submit your application: Applications can often be submitted online via the CIPRO website, streamlining the process.
- Pay the required fees: Fees are clearly outlined on the CIPRO website and vary based on the service.
- Monitor the progress: Track your application’s status through the online portal or contact CIPRO directly for updates.
Common Issues and Solutions
Expect potential delays. Addressing incomplete applications promptly minimizes processing time. Review all requirements carefully beforehand to avoid rejections.
- Incomplete applications: Double-check all submitted information for accuracy and completeness.
- Incorrect documentation: Ensure all documents are correctly formatted and meet the specified requirements.
- Payment issues: Verify payment confirmation and contact CIPRO if issues arise.
Utilize the CIPRO website’s FAQs and contact their support channels for assistance with any problems.
Contacting CIPRO
Find their contact information, including email addresses and phone numbers, on the official CIPRO website. Direct contact is frequently the fastest way to resolve specific issues.
Understanding the DTI’s Role in Ciprofloxacin Regulation
The Department of Trade, Industry and Competition (DTI) in South Africa oversees the registration and regulation of medicines, including ciprofloxacin, through its Medicines Control Council (MCC). This ensures that ciprofloxacin products meet quality, safety, and efficacy standards before they reach South African consumers.
Registration Process
The DTI, via the MCC, mandates a rigorous registration process for all ciprofloxacin-containing products. Manufacturers must submit comprehensive data packages demonstrating the drug’s quality, safety profile, and efficacy. This includes pre-clinical and clinical trial results, manufacturing details, and proposed labeling. The MCC then assesses this information to determine if the product meets the required standards for registration.
Post-Market Surveillance
Following registration, the DTI actively monitors ciprofloxacin’s safety and efficacy through post-market surveillance programs. This involves collecting and analyzing data on adverse drug reactions and product quality issues reported by healthcare professionals and patients. This continuous monitoring allows the DTI to quickly identify and address any potential safety concerns, implementing corrective actions as needed.
Import and Export Control
The DTI regulates the import and export of ciprofloxacin and related products, ensuring compliance with international regulations and South African standards. This prevents the entry of substandard or counterfeit medications and supports the integrity of the pharmaceutical supply chain.
Key Regulatory Aspects
| Aspect | DTI/MCC Responsibility |
|---|---|
| Quality Control | Ensures manufacturing processes meet GMP (Good Manufacturing Practice) standards. |
| Safety Monitoring | Tracks adverse events and takes appropriate action. |
| Pricing and Access | Plays a role in ensuring affordable access to ciprofloxacin. |
| Licensing and Permits | Grants necessary licenses for import, manufacture, and distribution. |
Reporting Adverse Events
Healthcare professionals and patients are encouraged to report any suspected adverse reactions to ciprofloxacin to the MCC. This reporting helps the DTI maintain a comprehensive understanding of the drug’s safety profile and allows for timely intervention if necessary. Contact details for reporting are readily available on the MCC website.
Ciprofloxacin’s Availability and Accessibility in South Africa
Ciprofloxacin is widely available in South Africa. You can find it at most pharmacies, both private and public, with a doctor’s prescription. Generic versions are readily available, making it a more affordable option compared to brand-name alternatives. However, accessibility remains a challenge for some. Rural areas often face stock shortages, and affordability continues to be an issue for individuals without medical aid.
Finding Ciprofloxacin: Practical Advice
To ensure you can access Ciprofloxacin, contact your doctor for a prescription. If you encounter difficulties sourcing it locally, consider contacting larger pharmacy chains or exploring online pharmacies that deliver nationwide – always ensure they’re licensed and reputable. For those with financial constraints, explore government-subsidized healthcare options or look into patient assistance programs offered by pharmaceutical companies. Regularly check with your local pharmacy regarding stock availability, especially in areas with potential supply issues. Public healthcare facilities also provide Ciprofloxacin, although waiting times may vary. Finally, always consult a healthcare professional before starting any medication.
Addressing Accessibility Gaps
Addressing access barriers requires collaboration. Pharmaceutical companies can improve distribution networks, especially to underserved communities. The government can enhance regulatory frameworks and support affordable generic drug production. Increased awareness campaigns can empower individuals to navigate the healthcare system effectively and advocate for their needs. Ultimately, ensuring equitable access to medication is a shared responsibility.
Pricing and Affordability of Ciprofloxacin in South African Pharmacies
Expect significant price variations across different South African pharmacies. Generic ciprofloxacin is generally more affordable than brand-name options. Check several pharmacies, both large chains and independent stores, to compare prices. Online pharmacy price comparison websites can also be helpful tools.
Factors Influencing Ciprofloxacin Prices
Pricing depends on the dosage form (tablets, suspension), quantity, and the pharmacy’s pricing structure. Discounts are sometimes available for larger quantities or with medical aid schemes. Always ask about potential discounts. Government subsidies may lower the cost for certain patients meeting specific criteria; inquire about these programs at your local pharmacy or clinic.
Tips for Affordable Access
Consider purchasing a generic version; it contains the same active ingredient as the brand name but costs less. Explore medical aid options if you have one, as they may cover a portion or all of the medication cost. If affordability remains a concern, consult your doctor or a pharmacist regarding potential assistance programs or lower-cost alternatives. Comparing prices from multiple sources is recommended before making a purchase.
Understanding Prescription Requirements
Ciprofloxacin requires a prescription in South Africa. Obtaining a prescription from a doctor is a legal requirement before purchasing the medication at any pharmacy. Do not attempt to purchase without a valid prescription.
Common Uses and Prescribing Practices of Ciprofloxacin in South Africa
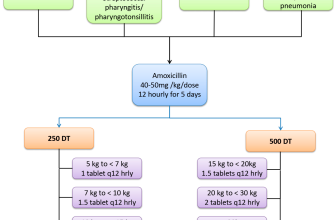
Ciprofloxacin, a fluoroquinolone antibiotic, enjoys widespread use in South Africa, primarily for treating bacterial infections. Doctors frequently prescribe it for urinary tract infections (UTIs), which represent a significant portion of its applications.
Respiratory Infections
Another common use is in treating respiratory infections like pneumonia and bronchitis, particularly those caused by susceptible bacteria. However, prescribing practices should consider local antibiotic resistance patterns; the increasing resistance of certain bacteria to ciprofloxacin is a significant concern.
Other Applications and Considerations
Beyond respiratory and urinary tract infections, ciprofloxacin finds application in treating skin and soft tissue infections, and some sexually transmitted infections. Doctors should always assess the patient’s medical history, considering potential drug interactions and contraindications, such as allergies or conditions impacting kidney or liver function. Appropriate dosage and duration of treatment are crucial, guided by the severity of infection and patient response.
Note: Self-medication is strongly discouraged. Always consult a healthcare professional for diagnosis and treatment of any infection.
Prescribing Guidelines and Monitoring
Adherence to national guidelines on antibiotic stewardship is vital. These guidelines aim to reduce the development and spread of antibiotic resistance. Monitoring treatment efficacy is also important; if symptoms persist or worsen after several days of treatment, a doctor should be consulted.
Safety Concerns and Side Effects Associated with Ciprofloxacin Use
Ciprofloxacin, while effective, carries potential risks. Always discuss these with your doctor before starting treatment.
Common side effects include:
- Nausea
- Diarrhea
- Vomiting
- Headache
- Dizziness
Less common, but serious, side effects require immediate medical attention:
- Severe allergic reactions (e.g., swelling of the face, throat, or tongue; difficulty breathing).
- Tendonitis or tendon rupture, particularly in the Achilles tendon. This risk increases with age and concurrent corticosteroid use.
- Peripheral neuropathy (numbness, tingling, or pain in the extremities).
- Seizures.
- Photosensitivity (increased sensitivity to sunlight).
- Clostridium difficile-associated diarrhea (a serious infection).
- Liver damage.
Specific precautions:
- Inform your doctor about all medications you are taking, including over-the-counter drugs and supplements, as interactions are possible.
- Avoid driving or operating machinery if you experience dizziness or other neurological side effects.
- Drink plenty of fluids to help prevent dehydration, especially if experiencing diarrhea.
- Protect yourself from excessive sun exposure.
- Report any unusual symptoms to your doctor immediately.
This information is not exhaustive. Consult your doctor or pharmacist for a complete list of potential side effects and drug interactions specific to your situation.
Reporting Adverse Events Related to Ciprofloxacin in South Africa
Suspect a Ciprofloxacin-related adverse event? Report it to the South African Health Products Regulatory Authority (SAHPRA). Use their online reporting system for quickest processing. Detailed information ensures accurate assessment.
Include specifics: Patient age, sex, medical history, Ciprofloxacin dosage, duration of treatment, and a thorough description of the adverse event, including onset, severity, and resolution (if applicable). Supporting documentation, such as lab results or medical notes, enhances the report.
SAHPRA’s website provides detailed guidance on reporting procedures. You’ll find templates and instructions to help you complete the report accurately and efficiently. Consider contacting SAHPRA directly for assistance if needed. Their contact details are readily available on their website.
Prompt reporting is crucial for detecting patterns and assessing the overall safety profile of Ciprofloxacin in South Africa. This information allows for timely interventions to protect public health.
Remember: While reporting suspected adverse events, protect patient confidentiality adhering to all relevant data protection legislation.