If you’re seeking reliable information about Amoxil, understanding its manufacturing origins is crucial. Amoxil, known generically as amoxicillin, is produced by several pharmaceutical companies, with GlaxoSmithKline being one of the most recognized manufacturers. This company ensures consistent quality and effectiveness, adhering strictly to regulatory standards. When choosing a medication, prioritize purchasing from reputable sources to guarantee you receive authentic products.
The production process of Amoxil involves multiple quality control measures, ensuring that each batch meets strict safety and efficacy guidelines. This commitment to quality helps in minimizing potential side effects while maximizing therapeutic benefits. Familiarizing yourself with the manufacturer’s background can significantly enhance your confidence in the medication you’re taking.
As you explore options for obtaining Amoxil, consider consulting with your healthcare provider, who can offer personalized advice on dosages and alternatives. Always opt for licensed pharmacies or medical professionals when procuring medication to safeguard your health and ensure that you receive the best possible care.
Amoxil Manufacturer: An Overview
Amoxil is manufactured by the pharmaceutical company GlaxoSmithKline (GSK). This antibiotic, known for treating various bacterial infections, is widely prescribed due to its effectiveness and safety profile. GSK employs rigorous quality controls and testing to ensure each batch meets high standards.
The active ingredient in Amoxil is amoxicillin, which belongs to the penicillin class of antibiotics. GSK utilizes both patented and generic formulations, making the medication accessible to a broader audience. The company engages in continuous research to enhance drug delivery and formulation techniques.
GSK has established a global distribution network, ensuring Amoxil is available in numerous countries. This reach allows healthcare providers to prescribe the medication confidently, knowing it is manufactured under strict regulatory guidelines. Additionally, GSK’s emphasis on patient education aids in effective use.
For those considering Amoxil, consulting a healthcare provider is recommended. This allows for personalized dosage and treatment plans based on specific infections and patient health status.
In summary, GSK’s commitment to quality and innovation in the manufacturing of Amoxil plays a significant role in its widespread use and trust among healthcare professionals and patients alike.
Key Players in Amoxil Production
The production of Amoxil, a widely used antibiotic, is led by several key manufacturers that ensure its availability and quality. Among these, GlaxoSmithKline (GSK) stands out as the original developer. They maintain rigorous quality control processes to ensure that Amoxil retains its efficacy against bacterial infections.
Teva Pharmaceuticals plays a significant role in the production of generic versions of Amoxil. Their extensive distribution network allows for widespread access, particularly in markets where affordability is a priority. Quality assurance measures are kept at the forefront of their manufacturing practices.
Dr. Reddy’s Laboratories is another notable manufacturer, focusing on providing competitive pricing and ensuring compliance with international standards. Their investment in research and development enables the continual enhancement of their Amoxil formulations.
Mylan, now part of Viatris, also contributes to Amoxil’s production landscape. They emphasize sustainability in their processes while delivering quality antibiotics to healthcare providers.
Finally, Sandoz, a Novartis division, ensures a consistent supply of Amoxil through strategic partnerships and robust manufacturing practices. Their global reach makes them a reliable source for both generic and branded versions of the drug.
By engaging with these manufacturers, healthcare professionals can ensure that patients receive effective treatment with Amoxil while supporting the continuity of high-quality antibiotic production.
Quality Control Standards in Amoxil Manufacturing
Implement strict adherence to Good Manufacturing Practices (GMP) throughout the production of Amoxil. This framework establishes guidelines ensuring that drugs are produced consistently and controlled to quality standards. Regular audits and inspections of manufacturing sites play a key role in maintaining compliance with these regulations.
Testing Protocols
Conduct extensive testing of raw materials and finished products. Utilize methods such as High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry to verify the chemical composition and purity of Amoxil. Stability testing should also be performed under various environmental conditions to guarantee the medication’s efficacy over its shelf life.
Documentation and Traceability
Maintain robust documentation practices to ensure traceability of each batch produced. Implement a system for recording the origin of raw materials, production processes, and quality control results. This not only aids in compliance but also facilitates quick identification and resolution of any issues that arise post-manufacturing.
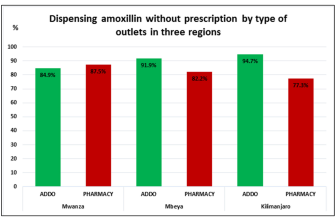
The Impact of Generic Versions on Amoxil Supply Chain
Generic versions of Amoxil significantly alter the supply chain dynamics. Manufacturers must adapt to increased competition from generics, leading to potential price reductions and changes in distribution strategies.
Firms should consider the following adjustments to maintain a robust supply chain:
- Cost Management: Implement strategies to reduce production costs through process optimization. Explore alternatives for raw materials that meet quality standards at a lower price.
- Partnerships: Establish collaborative relationships with suppliers. Solid partnerships can ensure a consistent supply of materials, reducing vulnerabilities to generic competition.
- Brand Differentiation: Focus on marketing the unique benefits of branded Amoxil. Highlighting specific indications, formulations, or delivery methods can strengthen brand loyalty.
- Regulatory Compliance: Stay updated on regulatory changes that may affect production and distribution. This proactive approach helps avoid potential disruptions.
- Patient Education: Engage in initiatives to educate patients about the importance of adherence to prescribed medications, countering potential shifts to generics.
Tracking market trends is essential. Analyze competitor strategies and market share regularly to adapt your approach to maintain supply chain integrity amidst the influx of generic options.
In conclusion, focusing on cost management, establishing partnerships, enhancing brand value, ensuring compliance, and increasing patient engagement will strengthen the supply chain in response to generic versions of Amoxil.